A 30 Year Plan to Move From Crude Oil to Synthetic Fuels
In a series of scholarly articles over the past year, a team led by Christodoulos Floudas, a professor of chemical and biological engineering at Princeton, evaluated scenarios in which the United States could power its vehicles with synthetic fuels rather than relying on oil. Floudas' team also analyzed the impact that synthetic fuel plants were likely to have on local areas and identified locations that would not overtax regional electric grids or water supplies.Am. Inst. Chemical Engineering article abstract
"The goal is to produce sufficient fuel and also to cut CO2 emissions, or the equivalent, by 50 percent," said Floudas, the Stephen C. Macaleer '63 Professor in Engineering and Applied Science. "The question was not only can it be done, but also can it be done in an economically attractive way. The answer is affirmative in both cases."
Accomplishing this would not be easy or quick, Floudas said. A realistic approach would call for a gradual implementation of synthetic fuel technology, and Floudas estimated it would take 30 to 40 years for the United States to fully adopt synthetic fuel. It also would not be cheap. He estimates the price tag at roughly $1.1 trillion for the entire system. _SD
Substituting for oil in the rubber industry
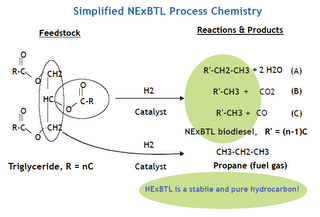
Using biomass, gas, coal, kerogens, bitumens, gas hydrates, etc. to produce substitutes for crude oil in fuels, high value chemicals, materials, lubricants, fertilisers, etc. is likely to grow more popular across the developed and emerging worlds.
Whichever feedstock is most available at affordable rates and in reliable quantities is likely to become more popular, at any given point in time. At this time, natural gas occupies that niche in North America. In the future, it may be coal or biomass -- or a combination of any of the above.
As high temperature gas cooled nuclear reactors become mass produced in modular form, the ability to convert any carbonaceous form into any hydrocarbon as needed, will become more affordable -- along with more abundant and reliable electrical power.
The impact of these substitute fuels and chemicals on "peak oil" will be mixed, and will depend largely upon governmental and inter-governmental regulations, as they develop in the near future. One possible paradoxical effect on future oil prices might involve the postponement of development of marginal oil deposits such as particular deep sea deposits, in anticipation of the development of more affordable oil substitutes.
Labels: green chemicals, synthetic gasoline